| 새로운 사진 | 신문속의 동물소식 | 신기한 동물이야기 | 동물의 소리 | 동물동화상 | 사진 올리기 | 사진 저작권 | English |
|---|
| 재미있는 동물사진 | 괴수/괴어/엽기 동물사진 | 동물이름사전 | 동물목록 | 바깥고리 | 창고입구 | 똑똑누리집 |
|---|
| 이미지 정보 | Original File Name: Giant Deer Liver Fluke (Fascioloides magna)_adult.jpg Resolution: 400x554 File Size: 67672 Bytes Upload Time: 2007:09:25 20:54:08 | |
| 올린이 | 이름 (메일주소): Unknown | |
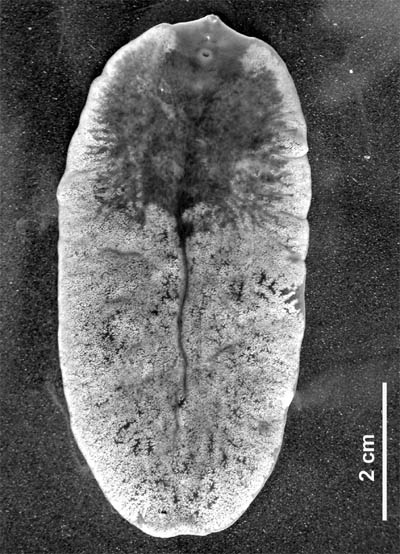
| 사진 제목 | Giant Liver Fluke (Fascioloides magna) - Wiki | |
 |
| Email : 카드 | 올린이 | 운영자 사진삭제 정보수정 Admin |
| 설명 | Giant Liver Fluke (Fascioloides magna) - Wiki
Fascioloides magna
Fascioloides magna (Bassi 1875), also known as giant liver fluke, large American liver fluke or deer fluke, is an important parasite of a variety of wild and domestic ruminants in North America and Europe. Adult flukes occur in the liver of the definitive host and feed on blood. Mature flukes measure 4 to 10 cm in length × 2 to 3.5 cm in width, and have an oval dorso-ventrally flattened body with oral and ventral sucker. The flukes are reddish-brown in colour and are covered by tegument. Similarly to that in other digenean trematodes, the life cycle includes intramolluscan phase in snails. History Fascioloides magna is essentially of North American origin but the parasite was introduced into Europe with imported game animals at the second half of the 19th century. In spite of being native to North America the fluke was first described in Italy. In 1875, Bassi observed massive deaths of red deer in the Royal Park near Torino, Italy. The signs were similar to well known fasciolosis in sheep. He named it Distomum magnum. The author believed that the parasite was introduced into the park in wapiti imported from USA in 1865. Most workers did not accept Bassi’s species because of his poor description. From 1882 to 1892, the fluke was recorded from different areas of the United States and described separately by many authors. Later, Stiles (1894) pointed out that the American findings are identical with species described previously by Bassi. Stiles made a complete morphological description of the adult fluke and named it Fasciola magna (Bassi 1875) Stiles 1894. In 1917, Ward showed that owing to the lack of the distinct anterior cone and the fact that vitellaria are confided to the region ventral to the intestinal branches, he established a new genus Fascioloides and rename it to Fascioloides magna (Bassi 1875) Ward 1917. In 1895, Stiles suggested that the life cycle of the fluke is very similar to Fasciola hepatica, i.e. it includes an aquatic snail as an intermediate host. He gave a comparative description of the egg and miracidium of the fluke. However, first reported intermediate hosts of F. magna were not published until 1930’s. The complete life cycle of F. magna, including a description of all the larval stages, was described by Swales (1935) in Canada . Life cycle The life cycle of F. magna is relatively complex and is similar to the development of the related fluke, F. hepatica. A detailed account of the F. magna life cycle was given by Swales (1935), Erhardov??-Kotrl?? (1971), and reviewed by Pybus (2001). Adult flukes occur in pairs or groups within a fibrous capsule in the liver parenchyma of the definitive host. Mature flukes release eggs which are collected in the cavity of the capsule. The capsule contains a great mass of eggs and has duct connections to bile ducts. The eggs are passed together with bile into the bile collecting system, enter the small intestine, and leave the definitive host along with the faeces. The eggs which are passed out in the faeces into the environment are undeveloped and undergo embryonation outside the host. Several physical-chemical factors, especially temperature, humidity and oxygen tension, are known to influence embryonation. During the embryonation of the egg, a larva called a miracidium develops from germinal cells. Fully developed miracidium releases the operculum of the egg using several proteases. The embryonation period varies from 27 to 44 days in natural conditions. Ciliated miracidia hatch in water and actively seek suitable intermediate hosts that are freshwater snails from family Lymnaeidae. After attaching to a suitable snail host, the miracidium penetrates into the snail body. After shedding its ciliated cell layer it is called a sporocyst. The sporocysts are found in the foot, the snail body, digestive glands, reproductive organs, and in the pulmonary sac of the snail. The sporocysts contain germinal cells that give rise to 1-6 mother rediae. Developed mother rediae are released from the sporocyst and migrate into digestive glands, renal organ, reproductive organs, and pulmonary sac of the snail body. Each mother redia can produce up to 10 daughter rediae. However, only 3 to 6 daughter rediae complete their development and leave the mother rediae. In turn, each daughter redia may produce 1-6 cercariae in experimentally infected snails and 16-22 cercariae under natural conditions. Cercariae emerge from the rediae and mature usually in digestive glands of the snail. Mature cercarie spontaneously emerge from the snail host and swim actively in water for up to two hours before encysting on vegetation. After encystment the flukes are called metacercariae. Development within the snail takes 40 to 69 days depending upon the temperature and the species of snail. The definitive host ingests vegetation containing the metacercariae. In the stomach and the intestine, the metacercariae are stimulated to emerge from the cyst (excystation). Newly excysted juvenile flukes penetrate the wall of the intestine and migrate in the abdominal cavity. Juvenile flukes penetrate the Glisson’s capsule of the liver and continue migrating in the liver tissue. Rarely juvenile flukes penetrate other organs, such as lungs or kidneys. In these organs, however, flukes do not survive and not attain maturity. In the liver, flukes migrate within the parenchyma to search another fluke. If the fluke meet another one, they stop moving, and the fibrous capsule is formed around them. In the capsule, the parasite completes its development and starts egg-laying. Prepatent period varies 3-7 months and is dependent on host species. Adult F. magna can survive in the liver of the host up to 7 years. Distribution Currently, F. magna occurs only in North America and Europe where suitable habitat exists and susceptible intermediate hosts are found. However, sporadic works reported unique appearance of the fluke in other continents. F. magna was found in imported animals in South Africa, Australia and Cuba. In all cases, infected animals (brahman heifer, ox, and elk, respectively) were imported from USA or Canada. North America During the 20th century, F. magna was reported in these American states: Arkansas, California, Colorado, Illinois, Iowa, Kansas, Louisiana, Michigan, Minnesota, Montana, New York, Oklahoma, Oregon, South Carolina, Texas, Washington, and Wisconsin. In Canada, the fluke was reported in Alberta, British Columbia, Ontario, and Quebec. Currently, F. magna is enzootic in five major areas: (1) the Great Lakes region; (2) the Gulf coast, lower Mississippi, and southern Atlantic seaboard; (3) northern Pacific coast; (4) the Rocky Mountain trench; and (5) northern Quebec and Labrador. However, within these broad ranges, actual presence of giant liver flukes varies from locally abundant to locally absent. Europe Fascioloides magna was first reported by Bassi in Torino, Italy. In spite of Bassi’s work, no other data concerning the occurrence of F. magna in Europe were reported until 1930’s . In the Czech territory, Ullrich reported the first appearance of F. magna in fallow deer as late as 1930. At the same time, Salomon (1932) diagnosed the fluke in one hunted red deer near G??rlitz (Saxony) in Germany. Other isolated findings of the fluke were recorded in Italy and Poland. From 1948 till 1961, sporadic occurrence of the parasite in red deer (Cervus elaphus), fallow deer (Dama dama) and roe deer (Capreolus capreolus) were reported by several authors in former Czechoslovakia. However, all reports were published on the basis of incident discoveries in hunted deer and no massive infections were documented . In 1960’s, a number of F. magna outbreaks in cervids were reported in some areas of former Czechoslovakia. The prevalence of infection varied from 70 to 80 % in red deer and maximum parasite burden was 144 worms. In addition, sudden deaths were documented in free or game ranging deer. The highest mortality was reported in free ranging roe deer in P??sek County in the South Bohemia of former Czechoslovakia. In the same region, moreover, the parasite was found in livers of slaughtered cattle.. Erhardov??-Kotrl?? (1971) confirmed red deer, fallow deer and roe deer as main definitive hosts of F. magna in Europe. In 1960’s, F. magna was enzootic in former Czechoslovakia in following four major areas: (1) ??esk?? Bud??jovice and T??ebo?? county, including Nov?? Hrady Mountains; (2) the area along the Vltava River on the Vltava-T??n hills near Hlubok?? and Bechyn??; (3) P??sek and Milevsko county; (4) the Brdy mountains and the H??ebeny mountains.In following years, F. magna was only reported from these areas. Recently, geographical distribution of F. magna in cervids was determined in the Czech Republic. The giant liver fluke was confirmed in the same areas as reported in 1960’s. However, seven new endemic areas of F. magna were discovered suggesting that the parasite is spreading in the Czech Republic. Moreover, the appearance of F. magna in the ??umava Mountains has epizootiological importance due to possibility of spread of the parasite into the German territory (Bavaria). During the last few years, a new European enzootic area has established in the Danube watershed in Central Europe. In 1988, F. magna was isolated from a 3-year old red deer female found dead near the Gab????kovo water plant at the Danube River in Slovakia. The parasite has spread through whole Slovakian Danube watershed. Soon after the Slovakian first report, F. magna was found in red deer in Hungarian parts of Danubian floodplain forests. The prevalence reported by the same authors was up to 90 %. F. magna infection of cervids is a considerable problem in northern part of Hungary (Szigetk??z) and the southern Danubian territory in the Gemenc area. Since the autumn of 2000, F. magna has been found in Austrian territory, east of Vienna. In years 2000-2001, the prevalence of the giant liver fluke in red deer in Austrian parts of Danube (east of Vienna) was 66.7 %. In January 2000, the giant liver fluke was diagnosed in hunted red deer in Baranja region in eastern Croatia. The parasite was probably introduced into Baranja region by natural deer migration from neighbouring Hungary. Regarding the origin of F. magna enzootic area in the Danube River watershed, it is essential to point out that cervids were not introduced into these localities, neither recently nor in the past. Origin of the F. magna population in Danubian floodplain forests in Central Europe remains therefore unclear. Definitive hosts Natural infections of F. magna occur primarily in cervids and bovids. Although many species are susceptible to infection, only a few cervid species contribute significantly to maintaining populations of the fluke. In North America, the common definitive hosts of the giant liver fluke are wapiti (Cervus elaphus canadensis), white-tailed deer (Odocoileus virginianus) and caribou (Rangifer tarandus). In Europe, F. magna occurs commonly in red deer (Cervus elaphus), fallow deer (Dama dama) and roe deer (Capreolus capreolus). Domestic ruminants are also susceptible to natural infection with F. magna. However, the infection is not patent, and domestic ruminants do not contribute to the propagation of the parasite in the environment. In North America, the giant liver fluke is commonly found in cattle, sheep and goats in areas where F. magna is enzootic in deer. In contrast, F. magna occurs rarely in domestic ruminants in Europe. The list of all natural definitive hosts of F. magna is presented in Table. The only indigenous primary definitive host of F. magna is white-tailed deer. This species has been parasitized by the fluke for the longest time in historical context. Wapiti and caribou are of Eurasian origin and entered North America during the Pleistocene epoch, and overlapped with white-tailed deer in some parts of North America. They might have encountered F. magna in these shared biotopes. Common name of species Latin name of species References NORTH AMERICA Bison Bison bison Black-tailed deer Odocoileus hemionus columbianus Caribou Rangifer tarandus Cattalo Bos taurus × Bison bison Cattle Bos taurus Collared peccary Dicotyles tajacu Goat Capra hircus Horse Equus caballus Llama Lama glama Moose Alces alces Mule deer Odocoileus hemionus hemionus Pig Sus scrofa var. domesticus Sheep Ovis aries Wapiti Cervus elaphus canadensis White-tailed deer Odocoileus virginianus Wild boar Sus scrofa Yak Bos grunniensis EUROPE Blue bull Bosephalus tragocamelus Cattle Bos taurus Fallow deer Dama dama Goat Capra hircus Horse Equus caballus Red deer Cervus elaphus Roe deer Capreolus capreolus Sambar Cervus unicolor Sheep Ovis aries Sika deer Sika nippon White-tailed deer Odocoileus virginianus Wild boar Sus scrofa ... http://en.wikipedia.org/wiki/Fascioloides_magna
| |||
| 댓글 |
| |||||||||||||
| 저작권 정보 | 사진의 저작권은 원저작자에게 있습니다. 동물그림창고는 동물관련 사진을 전시할 수 있는 공간만을 제공합니다.사진을 사용하고자 할 경우에는 저작권자와 협의하시기 바랍니다. |
|
|
|
| |||||||
| CopyLeft © since 1995, 동물그림창고. All rights may be reserved. | ||||||||
Stats